ECHA Biocidal Products Regulation
The Biocidal Products Committee (BPC) prepares the opinions of the European Chemical Agency (ECHA) related to several processes under the Biocidal Products Regulation. Substances, which were on the market before 14 May 2000 are referred to as existing active substances.
During the approval process the evaluating competent authority may conclude that the active substance meets the criteria for substitution of Article 10(1) of the Biocidal Products Regulation (BPR) and is therefore a potential candidate for substitution. The objective of this provision is to identify substances of particular concern to public health or the environment and to make sure that these substances are phased-out and replaced by more suitable alternatives over time.
ECHA’s Biocidal Products Committee has for the first time supported the approval of substances that meet the BPR exclusion criteria.
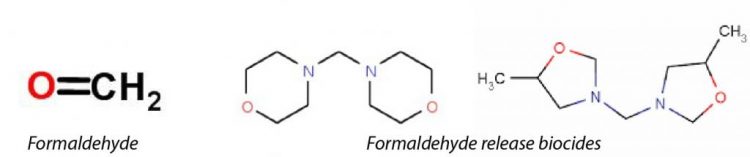
Formaldehyde releases biocides
Water mix metalworking fluids are subject to contamination by bacteria and fungi, and the control of this is an essential part of good fluid maintenance. The use of preservatives both within the formulation and tank-side treatment plays a significant contribution in the protection of potentially harmful microbes that could cause health problems for workers.
A large proportion of bactericides on the market today are classed as formaldehyde releasing biocides which means that under specific conditions they release small amounts of formaldehyde – this is their mode of action in the presence of bacteria. Although they are effective as a biocide their use may become restricted or unfavourable due to potential changes in legislation.
A decision by the ECHA (European Chemicals Agency) was made to re-classify formaldehyde as a category 1b H350 carcinogen and category 2 mutagen in June 2015.
It has also been proposed by the ECHA Risk Assessment Committee (RAC) that formaldehyde release biocides should be classified the same as formaldehyde because formaldehyde is released when these substances come into contact under favorable conditions (i.e. interaction with microorganisms).
ECHA’s Biocidal Products Committee has for the first time supported the approval of substances that meet the BPR exclusion criteria and has so far highlighted two formaldehyde release biocides (MBO and HPT) and recommended them as a candidate for substitution. It is highly likely that this proposal will affect a whole group of similar acting biocides of which there are at least ten.
The potential outcome could therefore result in the elimination from the market of an entire group of active substances.
Whereas the re-classification of formaldehyde is not being contested; concerns have been raised by biocide and metalworking fluid manufacturers through a ‘Formaldehyde Biocide Interest Group’ (FABI). Their main concern is the rational justification in applying the same classification of formaldehyde to the parent’ formaldehyde releaser and the small amount of free formaldehyde generated in the preserved product. The argument being that there is simply not enough evidence to suggest that they present a hazard to human health.

Timescales
Despite the opposition the first formaldehyde releasing biocide will be reclassified as carcinogen 1B in December 2018. It is likely that all other formaldehyde release biocides will follow suit on a step by step basis most likely until 2025.
Impact
- Some formaldehyde containing products maybe withdrawn from the market.
- An increased severity of the product label may be incurred.
- Extra measures maybe required to ensure formaldehyde levels in the workplace atmosphere are within permissible levels. The Scientific Committee for Occupational Exposure Limits (SCOEL) has proposed 300 ppb for formaldehyde and there is strong likelihood that this will be agreed by ECHA. However, it should be noted that the average working place concentration is well below this limit (approx. 50 ppb) when using formaldehyde releasing biocides.
- There is a risk that MWF products reformulated could be less effective and / or more expensive as alternative biocides and methodology are more costly.
Reaction
It is increasingly likely that the MWF market will move away from the use of formaldehyde based biocides. In some cases this is already happening but this is likely to increase from now and during 2018.
Increased awareness and fluid condition management of soluble metalworking fluids will be required to limit the impact any loss in biocidal efficacy. It remains critically important to maintain MWF’s in use to comply with COSHH 2002 requirements.
What can users of MWFs do?
- Consult your Q8Oils representative.
- Review alternative formaldehyde free products with a view to future substitution.
Q8Oils’ response
At Q8Oils we are already planning for the proposed legislation requirements and have developed formaldehyde free soluble MWF product ranges: Q8 Berlioz and Q8 Brunel.
These have been carefully designed with innovative raw materials to provide the optimum bio-stability within future legislation requirements.
Q8Oils will continue to provide metalworking fluids containing formaldehyde release biocides for the present time.
Q8Oils is an active member of the United Kingdom Lubricants Association (UKLA) and key member of the HSE MWF guidance panel for the management of metalworking fluids. We will continue to monitor progress and are ready to fully comply and adapt to any proposed legislation changes. We also remain fully committed to keeping customers informed and assist them in adapting to the changes.