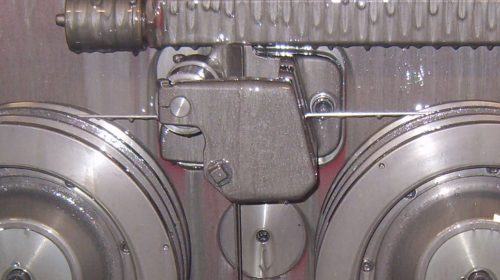
Cobalt is an important component in the manufacture of cemented tungsten carbide. Powdered cobalt is mixed with the irregular shaped tungsten carbide particles in a proportion of 3% to 30% of the total weight for sintering. During the sintering process the cobalt melts and forms a matrix that fills the inter-granular voids, binding the grains in place. The carbide tips or solid carbide blanks are now ready for grinding into their final profile.
The cobalt that is released into metalworking fluid (MWF) as a result of the shaping and sharpening processes may dissolve and circulate in the fluid or become sediment at the bottom of the filter into which it was released. Under corrosive environmental conditions cobalt is spontaneously released in a reaction that is commonly referred to as leaching. Leaching describes the dissolution of the cobalt binder matrix at the surface brought about by chemical or electrochemical reaction between the carbide surfaces and the environment. The tungsten carbide grains are unaffected. However, with the loss of the binder metal, the structure collapses providing access for the corrosive atmosphere or liquid to attack new surfaces, repeating the leaching process.
Cobalt is a ‘heavy metal’ with a specific gravity of 8.9 at 20?C. Cobalt is toxic and can cause poisoning if it is swallowed, breathed in, or absorbed through the skin. Nonradioactive cobalt has not been found to cause cancer in humans or in animals following exposure in the food or water. Cancer has been shown, however, in animals who breathed cobalt, or when cobalt was placed directly into the muscle or under the skin. Based on the animal data, the International Agency for Research on Cancer (IARC) has determined that cobalt metal with tungsten carbide is Probably carcinogenic to humans, and classified it as 2A.
For most people, food is the largest source of cobalt intake. The average person consumes about 11 micrograms of cobalt a day in their diet. If you work in metal mining, smelting, and refining, in industries that make or use cutting or grinding tools, or in other industries that produce or use cobalt metal and cobalt compounds, then you may be exposed to higher levels of cobalt. If good industrial hygiene is practiced, such as the use of exhaust systems in the workplace, exposure can be reduced to safe levels. Industrial exposure results mainly from breathing cobalt-containing dust.
When too much cobalt is taken into your body, however, harmful health effects can occur. Workers who breathed air containing 0.038 mg cobalt/m3 (about 100,000 times the concentration normally found in ambient air) for 6 hours had trouble breathing. Serious effects on the lungs, including asthma, pneumonia, and wheezing, have been found in people exposed to 0.005 mg cobalt/m3 while working with hard metal, a cobalt tungsten carbide alloy. People exposed to 0.007 mg cobalt/m3 at work have also developed allergies to cobalt that resulted in asthma and skin rashes.
To inhibit cobalt leaching it is necessary to protect the grinding operation from moderate to highly corrosive environments. A protective environment can be achieved using a MWF that provides adequate heat dissipation, is within a pH range 8 to 10, and contains special additives designed to inhibit the dissolution of cobalt in water.